Update November 2022
This Rejigit writer fell victim to Covid-19 and it proved difficult to find a pharmacy which is part of the New Zealand government's Paxlovid antiviral roll-out. Unichem Bishopdale Pharmacy in Christchurch New Zealand (phone 03 3598302) is one that can dispense the pharmacist only medication and they were very helpful and efficient. The pharmacist will need to access blood test results from within the previous six months in order to confirm renal function is normal. The medication came with a note indicating that diarrhea is a possibility and that proved to be an understatement. The government fully funded medication can also be couriered to one's home at no cost.
.
Update September 2022
New Zealand's Pharmac medicines agency has announced that a preventative Covid-19 medicine will be made available to protect people from getting COVID-19 or from becoming very sick if they do get it and it may reduce risk of being admitted to hospital.
Evusheld is a combination of Tixagevimab and Cilgavimab and it will be available to those who have a weakened immune system (immunocompromised). The medicine is mostly used before you get COVID-19 rather than after but, in some circumstances, it may be used as a treatment.
.
16 July 2022
The New Zealand Pharmaceutical Management Agency (Pharmac) has recently announced that it has widened access to three antiviral medicines to treat COVID-19 with effect from 18 July 2022.
This could be good news for several eligible groups of people including those who are aged 75 and older and the antiviral medicines are free of cost courtesy of the New Zealand government.
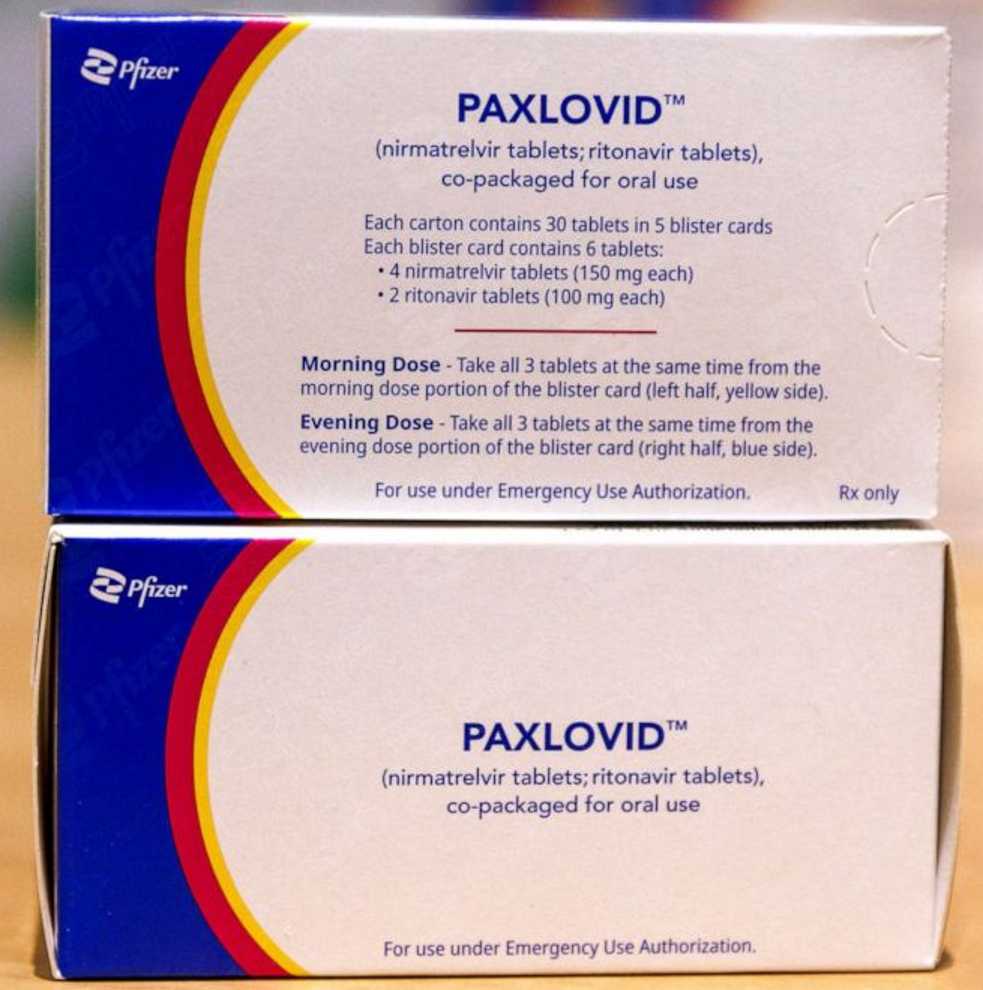
One of the available antivirals is Paxlovid. A combination of nirmatrelvir which inhibits an enzyme which the COVID virus and its several variants requires in order to make functional virus particles and ritonavir which essentially inhibits nirmatrlvir’s metabolism in the liver in order that it doesn’t move out of the body quickly and which allows the antiviral to remain effective for longer. Paxlovid is thought to be up to 90% effective if taken early in the course of an illness. A usual course of Paxlovid comprises three Paxlovid pills twice daily for five days for a full course of 30 pills.
Availability of the antivrals is subject to prescription by an appropriate medical practitioner.
.
The following is an abridged version of Pharmac’s July 14, 2022 announcement, the full version of which is available at https://pharmac.govt.nz/news-and-resources/consultations-and-decisions/july-2022-access-criteria-updated-covid-19-antivirals/
....Nirmatrelvir with Ritonavir (supplied under the brand name Paxlovid).
....Molnupiravir (supplied under the brand name Lagevrio).
....Remdesivir (supplied under the brand name Veklury).
The treatments are currently available in New Zealand and are being used in the treatment of early COVID-19 for people at risk of severe illness from COVID-19 infection. Criteria changes implemented from 18 July 2022 will make these treatments available to a larger priority population of people at risk of severe illness from COVID-19 in New Zealand.
The updated criteria widens access to antiviral treatments to additional groups of people who are at increased risk of severe COVID-19 including people aged 75 years and older.
No changes have been made to the distribution arrangements for these treatments at this time. More information about New Zealand’s COVID-19 treatments portfolio, availability of the treatments and how to access them is available here https://pharmac.govt.nz/news-and-resources/covid19/treatcovid/
The following Access Criteria will apply to nirmatrelvir with ritonavir (Paxlovid), molnupiravir (Lagevrio) and remdesivir (Veklury). Prescriptions must be endorsed by the prescriber confirming that the patient meets the Access Criteria.
Access criteria – from any relevant practitioner.
Approvals are valid for patients where the prescribing clinician confirms the patient meets the following criteria and has endorsed the prescription accordingly:
All of the following:
Patient has confirmed (or probable) symptomatic COVID-19, or has symptoms consistent with COVID-19 and is a household contact of a positive case;
AND
Patient’s symptoms started within the last 5 days (if considering nirmatrelvir with ritonavir or molnupiravir) or within the last 7 days (if considering remdesivir);
AND
Patient does not require supplemental oxygen#;
AND
ANY of the following:
The patient meets ONE of the following:
Patient is immunocompromised* and not expected to reliably mount an adequate immune response to COVID-19 vaccination or SARS-CoV-2 infection, regardless of vaccination status; or
Patient has Down syndrome; or
Patient has sickle cell disease; or
Patient has had a previous admission to ICU directly as a result of COVID-19; or
Patient is aged 75 years or over
Information for people with COVID-19
If you have, or suspect you have COVID-19 and are at high risk of developing severe illness from COVID-19, test early and please get in touch with your health care provider. They are best placed to let you know what your treatment options are. Treatments must be started within short time frames from onset of symptoms. You can find more information on the Ministry of Health website here https://www.health.govt.nz/covid-19-novel-coronavirus